Structural Basis for Lifetime Regulation of Mitochondrial Proteins

Assistant Professor, Graduate School of Science, Kyoto University
http://kuchem.kyoto-u.ac.jp/kozo/index_en.html
researchmap: https://researchmap.jp/kei_okatsu
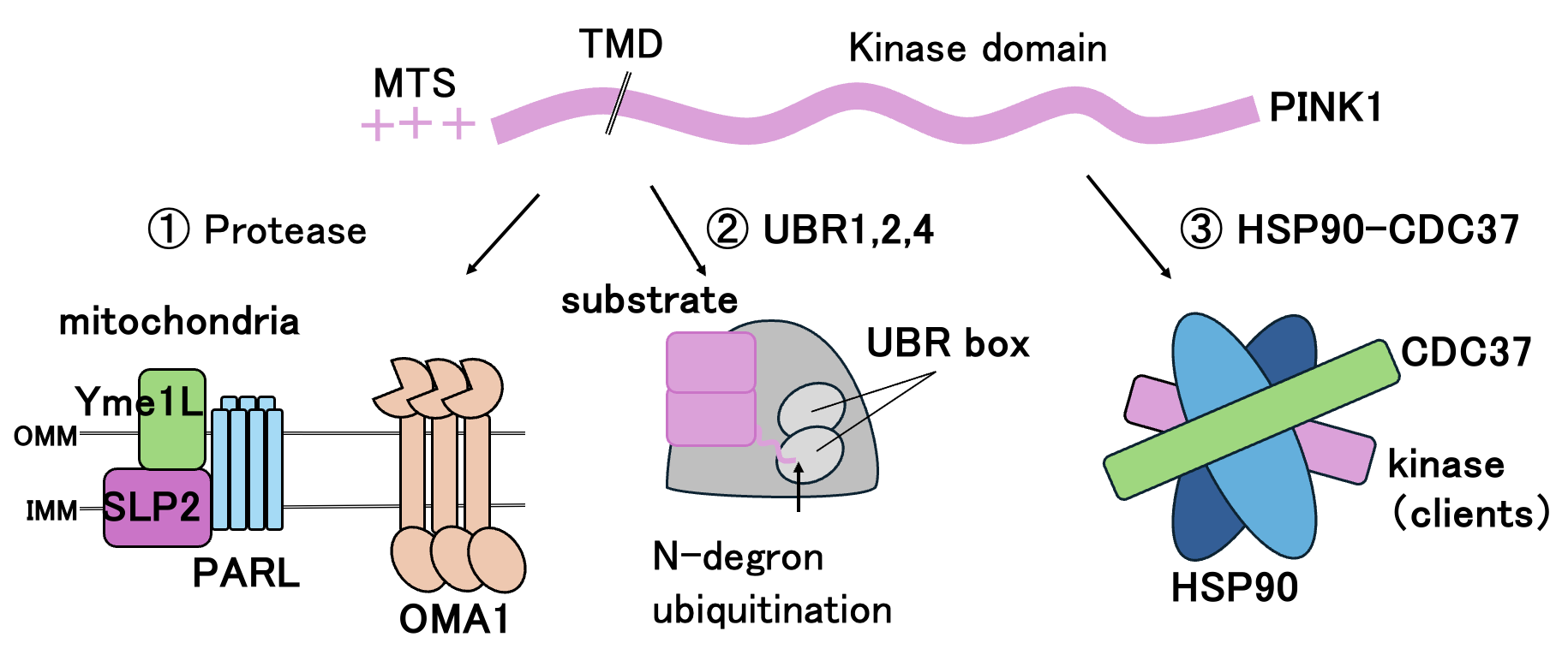
Mitochondrial membrane potential affects energy production and protein transport into mitochondria. One of the proteins that fluctuates with decreased mitochondrial membrane potential is PINK1, which has an N-terminal mitochondrial targeting signal and transmembrane domain, and a C-terminal serine/threonine protein kinase domain. In normal mitochondria, PINK1 is cleaved at the transmembrane domain by mitochondrial inner membrane proteases. The exposed N-terminal degron is recognized by a ubiquitin ligase UBR and ubiquitinated for leading to proteasomal degradation. Folding of PINK1 has been implicated in the kinase-chaperone complex. Although the intracellular dynamics of PINK1 are well understood, the structural basis of its degradation and stabilization remains to be elucidated. PINK1 regulatory proteins also regulate proteins other than PINK1. In this study, analyzing PINK1 as a model substrate, we will perform structural analysis (X-ray crystallography and cryo-EM single particle analysis), biochemical and physicochemical methods to elucidate the details of the degradation and stabilization processes. Thereby deepening our understanding of the molecular mechanisms that regulate protein lifetime.
- Okatsu K., Sato Y., Yamano K., Matsuda N., Negishi L., Takahashi A., Yamagata A., Goto-Ito S., Mishima M., Ito Y., Oka T., Tanaka K., & Fukai S. (2018) Structural insights into ubiquitin phosphorylation by PINK1. Sci Rep. 10.1038/s41598-018-28656-8
- Okatsu K., Koyano F., Kimura M., Kosako H., Saeki Y., Tanaka K., & Matsuda N. (2015) Phosphorylated ubiquitin chain is the genuine Parkin receptor. Journal of Cell Biology. 10.1083/jcb.201410050
- Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., Endo T., Fon E. A., Trempe J. F., Saeki Y., Tanaka K., & Matsuda N. (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 10.1038/nature13392
- Okatsu K., Oka T., Iguchi M., Imamura K., Kosako H., Tani N., Kimura M., Go E., Koyano F., Funayama M., Shiba-Fukushima K., Sato S., Shimizu H., Fukunaga Y., Taniguchi H., Komatsu M., Hattori N., Mihara K., Tanaka K., & Matsuda N. (2012) PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun. 10.1038/ncomms2016
- Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F., Kimura M., Komatsu M., Hattori N., & Tanaka K. (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. Journal of Cell Biology. 10.1083/jcb.200910140
