A02
Molecular mechanisms underlying turnover of lysosomal proteins and their role in aging

Shuhei Nakamura
Professor, Department of Biochemistry, Nara Medical University
https://bioch.naramed-u.ac.jp/index-e.html
researchmap: https://researchmap.jp/shuhei
Professor, Department of Biochemistry, Nara Medical University
https://bioch.naramed-u.ac.jp/index-e.html
researchmap: https://researchmap.jp/shuhei
Abstract
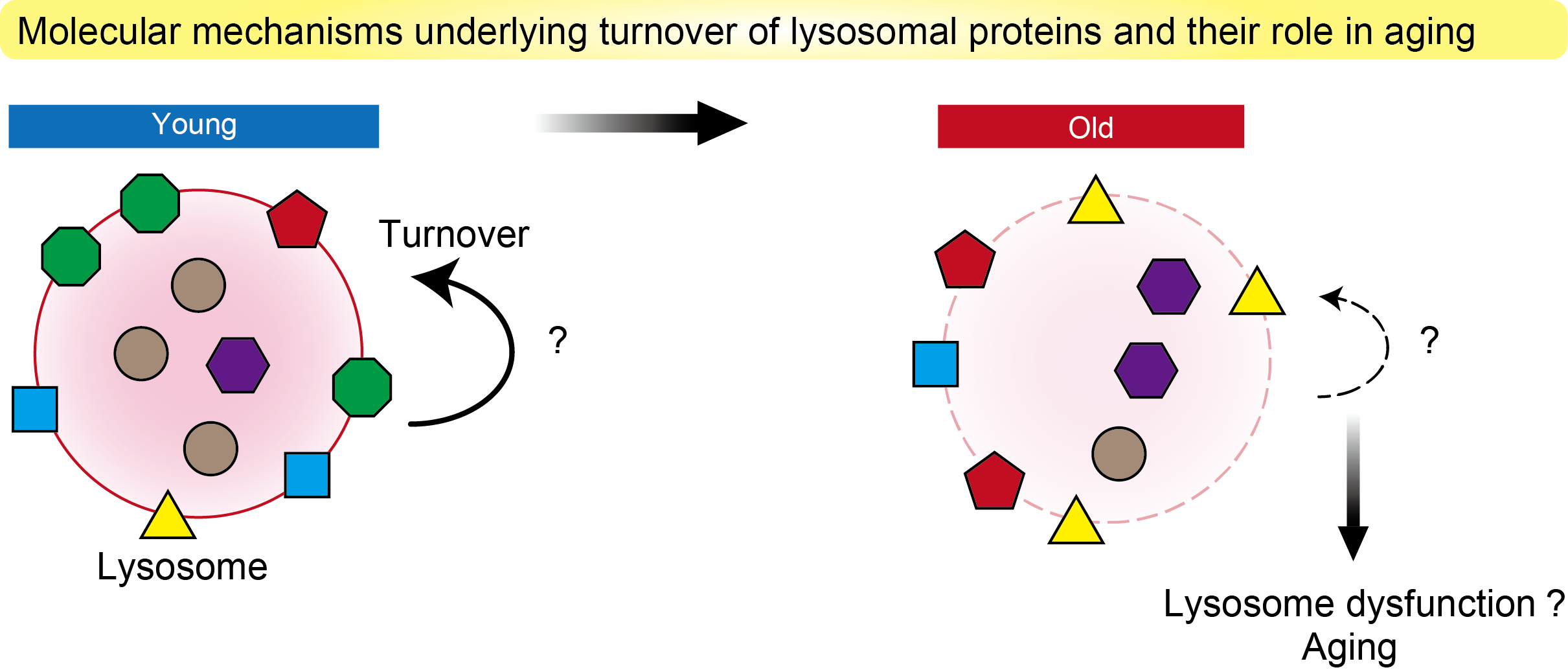
Lysosomes play an essential role in cellular and organismal homeostasis, through their function as degradative organelles and a signaling hub. However, how lysosomal homeostasis is maintained is not well understood. Especially, how the turnover of more than 150 lysosomal membrane proteins and 50 hydrolytic enzymes is regulated remains largely elusive. It has also been reported that lysosomal function declines with age, but the underlying mechanism for this decline and its causal relationship to aging are unclear. In this study, we will clarify the molecular mechanisms of lysosomal protein turnover and its role in aging.
- Cui M., Yamano K., Yamamoto K., Yamamoto-Imoto H., Minami S., Yamamoto T., Matsui S., Kaminishi T., Shima T., Ogura M., Tsuchiya M., Nishino K., Layden B. T., Kato H., Ogawa H., Oki S., Okada Y., Isaka Y., Kosako H., Matsuda N., Yoshimori T., & Nakamura S. (2024) HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence. Proc Natl Acad Sci U S A. 10.1073/pnas.2306454120
- Ogura M., Kaminishi T., Shima T., Torigata M., Bekku N., Tabata K., Minami S., Nishino K., Nezu A., Hamasaki M., Kosako H., Yoshimori T., & Nakamura S. (2023) Microautophagy regulated by STK38 and GABARAPs is essential to repair lysosomes and prevent aging . EMBO Rep. 10.15252/embr.202357300
- Shioda T., Takahashi I., Ikenaka K., Fujita N., Kanki T., Oka T., Mochizuki H., Antebi A., Yoshimori T., & Nakamura S. (2023) Neuronal MML-1/MXL-2 regulates systemic aging via glutamate transporter and cell nonautonomous autophagic and peroxidase activity. Proc Natl Acad Sci U S A. 10.1073/pnas.2221553120
- Nakamura S., Shigeyama S., Minami S., Shima T., Akayama S., Matsuda T., Esposito A., Napolitano G., Kuma A., Namba-Hamano T., Nakamura J., Yamamoto K., Sasai M., Tokumura A., Miyamoto M., Oe Y., Fujita T., Terawaki S., Takahashi A., Hamasaki M., Yamamoto M., Okada Y., Komatsu M., Nagai T., Takabatake Y., Xu H., Isaka Y., Ballabio A., & Yoshimori T. (2020) LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat Cell Biol. 22, 1252–1263
- Nakamura S., Oba M., Suzuki M., Takahashi A., Yamamuro T., Fujiwara M., Ikenaka K., Minami S., Tabata N., Yamamoto K., Kubo S., Tokumura A., Akamatsu K., Miyazaki Y., Kawabata T., Hamasaki M., Fukui K., Sango K., Watanabe Y., Takabatake Y., Kitajima T. S., Okada Y., Mochizuki H., Isaka Y., Antebi A., & Yoshimori T. (2019) Suppression of autophagic activity by Rubicon is a signature of aging. Nat Commun. 10.1038/s41467-019-08729-6
