A New Substrate Recognition Mechanism for Ubiquitination by Hetero-Ubiquitin Ligases

Associate professor, Department of Chemistry, Faculty of Science, Kyushu University
http://www.scc.kyushu-u.ac.jp/horilab/en/
researchmap: https://researchmap.jp/ymkana
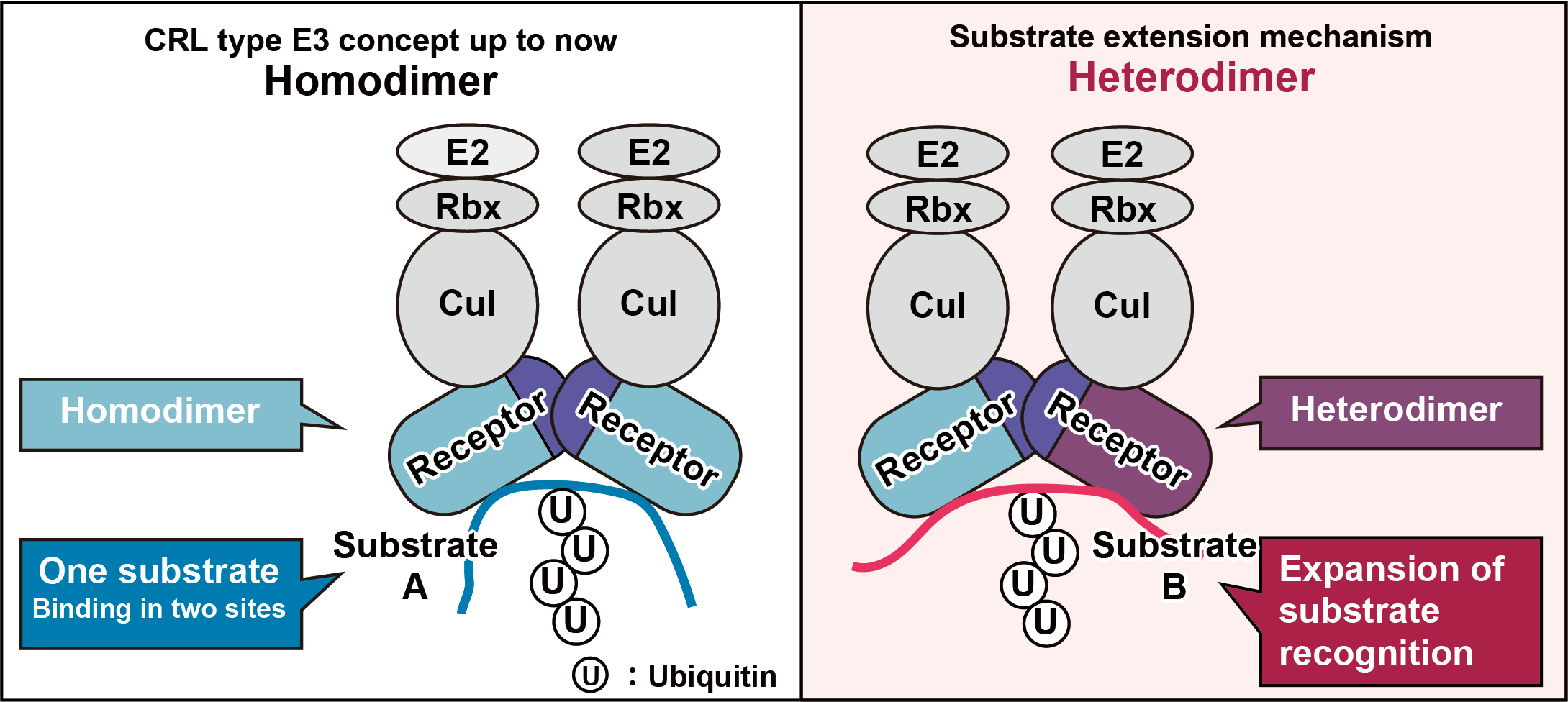
Ubiquitin-mediated proteolysis has been implicated in the quantitative regulation of various signaling factors and cell cycle regulators, and has been shown to be involved in a wide range of biological phenomena. According to the decoding of human genome information, there are approximately 600 human ubiquitin ligases (E3s) based on their domain structures, but there are reported to be approximately 9,200 proteins that are ubiquitinated in human cells. Therefore, by simple calculation, one E3 must ubiquitinate more than 15 types of proteins, but there are few examples of a single E3 ubiquitinating more than 15 molecules, suggesting that E3 is somehow expanding its substrate recognition methods. Given that life often employs strategies to increase diversity by exploiting combinations (e.g., reconstitution of immunoglobulin genes), we hypothesized that E3s may be expanding their diversity in a similar manner. In preliminary studies, we found that the substrate receptor proteins of CRL-type E3 form a heterodimer and that changing their combination may expand the diversity of substrate recognition. This study will elucidate the impact of this novel mechanism of substrate selectivity expansion on protein lifetime determination. This study will provide a new understanding of the complexity and diversity of the proteolytic mechanism of ubiquitination, which may have future applications in disease therapy and cell regulation.
- Yumimoto K., Sugiyama S., Motomura S., Takahashi D., & Nakayama K. I. (2023) Molecular evolution of Keap1 was essential for adaptation of vertebrates to terrestrial life. Sci Adv. 10.1126/sciadv.adg2379
- Yumimoto K., & Nakayama K. I. (2020) Recent insight into the role of FBXW7 as a tumor suppressor. Semin Cancer Biol. 67, 1–15
- Yumimoto K., Akiyoshi S., Ueo H., Sagara Y., Onoyama I., Ueo H., Ohno S., Mori M., Mimori K., & Nakayama K. I. (2015) F-box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner. Journal of Clinical Investigation. 10.1172/JCI78782
- Hirano A., Yumimoto K., Tsunematsu R., Matsumoto M., Oyama M., Kozuka-Hata H., Nakagawa T., Lanjakornsiripan D., Nakayama K. I., & Fukada Y. (2013) FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell. 10.1016/j.cell.2013.01.054
