Analysis of the p97 and its cofactors response to SUMO and branched ubiquitin chains

Associate Professor, Faculty of Engineering, Tottori University
researchmap: https://researchmap.jp/yusuke-sato
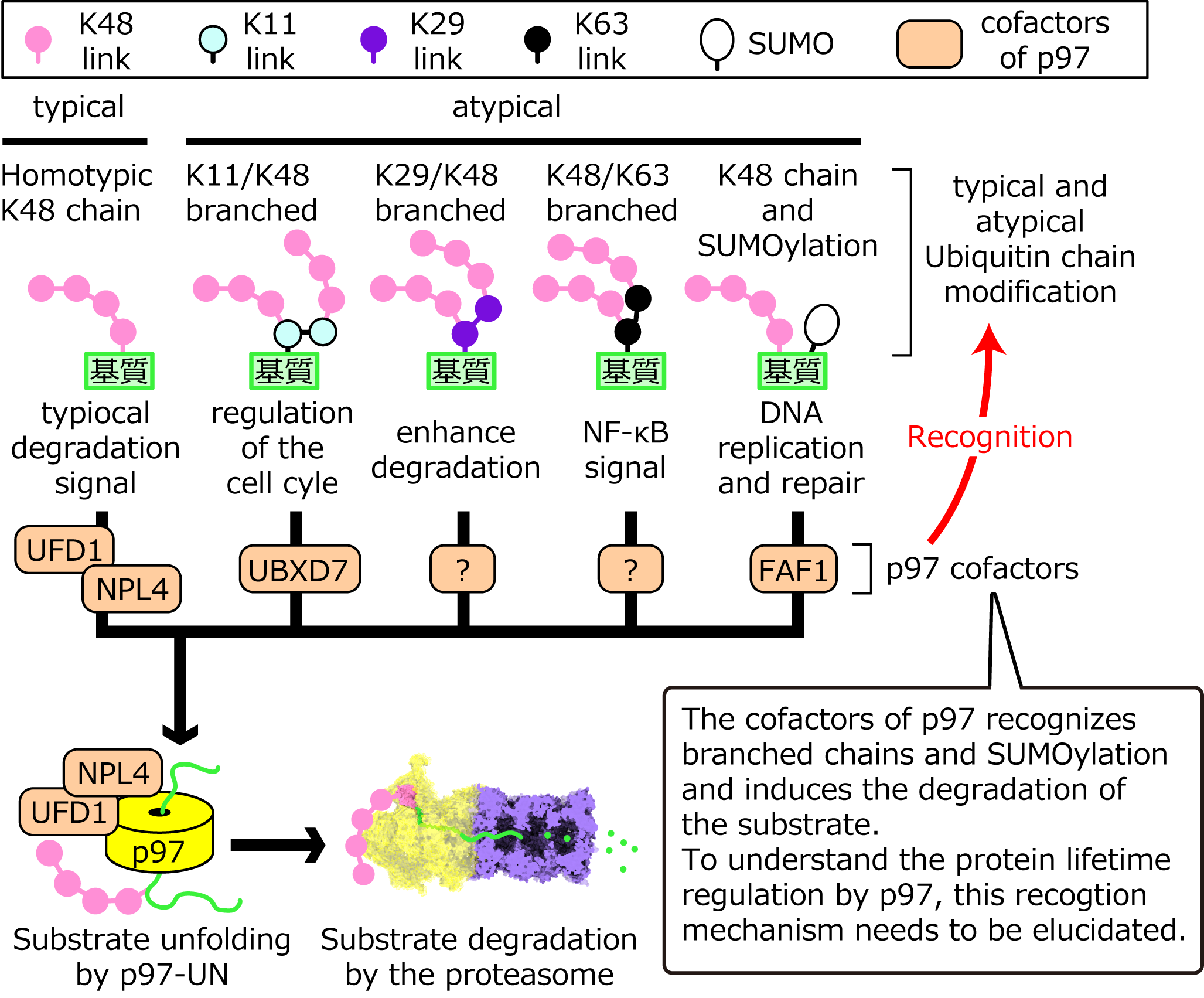
p97 plays a central role in determining protein lifetime, by unfolding polyubiquitinated substrates and leading to proteasomal degradation. About 30 different cofactors regulate the function of p97. For example, one of the p97 cofactors, the UFD1-NPL4 heterodimer (UN), specifically binds to K48-linked ubiquitin chains (K48 chains) and is essential for unfolding of polyubiquitinated substrates by p97. On the other hand, it has been reported that the p97 cofactors UBXD7 and FAF1 promote the degradation of substrates modified by K11/K48 branched ubiquitin chains and SUMO, respectively. However, the mechanism by which these cofactors enhance the activity of p97 for substrates modified by branched chains or SUMO is largely unknown. In this study, we will synthesize substrates modified by branched chain or SUMO in vitro. We will then analyze how the cofactors regulate the p97 activities to the obtained substrates and clarify the mechanism of protein lifetime determination by atypical ubiquitin chains and SUMOylation in cells.
- Sato Y. (2022) Structural basis for the linkage specificity of ubiquitin-binding domain and deubiquitinase. The Journal of Biochemistry. 172, 1–7
- Sato Y., Tsuchiya H., Yamagata A., Okatsu K., Tanaka K., Saeki Y., & Fukai S. (2019) Structural insights into ubiquitin recognition and Ufd1 interaction of Npl4. Nat Commun. 10, 5708
- Sato Y., Okatsu K., Saeki Y., Yamano K., Matsuda N., Kaiho A., Yamagata A., Goto-Ito S., Ishikawa M., Hashimoto Y., Tanaka K., & Fukai S. (2017) Structural basis for specific cleavage of Lys6-linked polyubiquitin chains by USP30. Nat Struct Mol Biol. 24, 911–919
- Sato Y., Goto E., Shibata Y., Kubota Y., Yamagata A., Goto-Ito S., Kubota K., Inoue J., Takekawa M., Tokunaga F., & Fukai S. (2015) Structures of CYLD USP with Met1- or Lys63-linked diubiquitin reveal mechanisms for dual specificity. Nat Struct Mol Biol. 22, 222–229
- Sato Y., Yoshikawa A., Yamagata A., Mimura H., Yamashita M., Ookata K., Nureki O., Iwai K., Komada M., & Fukai S. (2008) Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature. 455, 358–362
