Alteration of protein lifetime in vascular stenosis

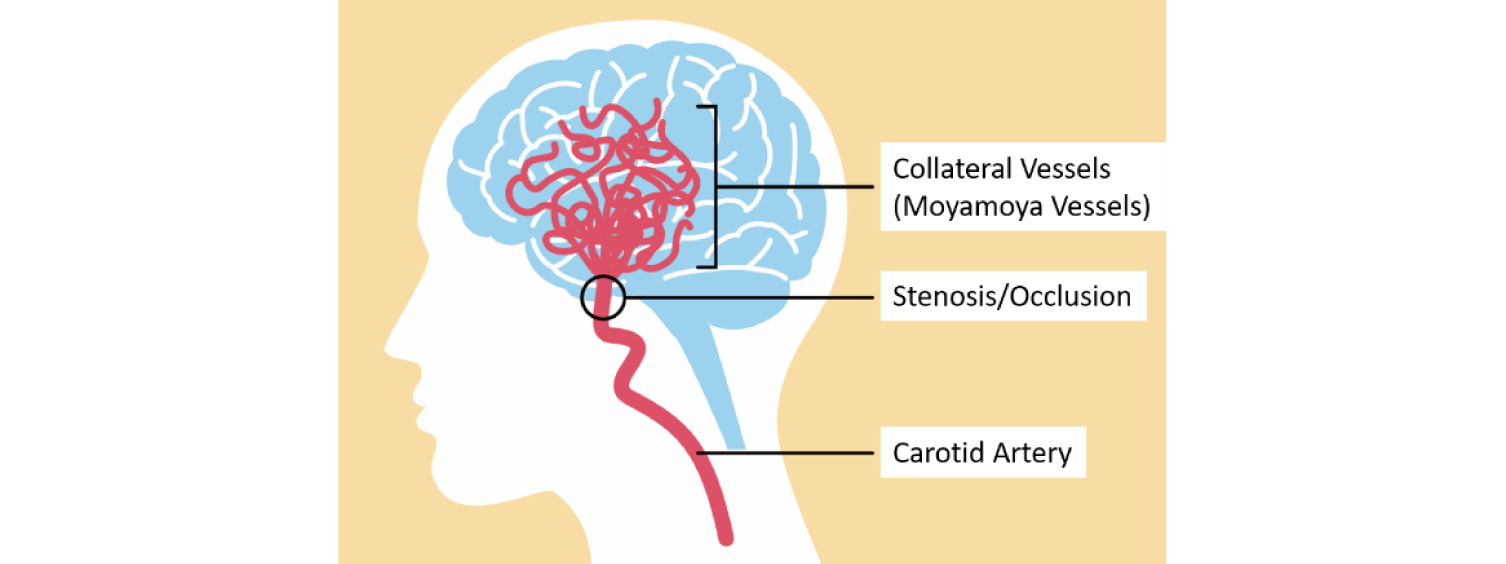
Moyamoya disease (MMD) is an intractable cerebrovascular disease that initiates with cerebral vascular stenosis. Mechanism underlying the onset and progression of MMD has not yet been elucidated, but it is confirmed that genetic factors play a significant role. We have succeeded in the first molecular cloning of the gene responsible for MMD and have been elucidating its activity and function. The moyamoya disease responsible gene that we named mysterin (also known as RNF213 or ALO17) encodes a large intracellular protein. Mysterin protein is a unique enzyme harboring dynein-type six tandem AAA+ ATPase domains and two ubiquitin ligase domains. Most of their activities were first detected by our group. We have also found that mysterin is involved in intracellular fat metabolism through these activities. On the other hand, several groups have recently reported that mysterin can attack pathogenic bacteria that infect cells. At the time of the establishment of this Shin-biology area, it is not clear how the metabolic control and infection defense functions of mysterin could be explained in a unified manner, and how these functions (and their abnormalities) could be related to MMD. We have identified that mysterin ubiquitylates different protein subsets under various physiological and pathological conditions. Downstream of these processes, specific subproteome lifespan regulation is thought to occur. In this study, we will elucidate the reality and mechanism of these processes, as well as the mechanisms by which these large-scale alterations in protein lifetime lead to MMD and related stenotic disorders.
- Sugihara M, *Morito D et al. AAA+ ATPase/ ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J Cell Biol 218, 949-960 (2019)
- Kotani Y, *Morito D et al. Alternative exon skipping biases substrate preference of the deubiquitylase USP15 for mysterin/RNF213, the moyamoya disease susceptibility factor. Sci Rep 7, 44293 (2017)
- Kotani Y, *Morito D et al. Neuromuscular regulation in zebrafish by a large AAA+ ATPase/ubiquitin ligase, mysterin/RNF213. Sci Rep 5, 16161(2015)
- Morito D et al. Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state. Sci Rep 4, 4442 (2014)
- Liu W, Morito D (co-first) et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLOS ONE 6, e22542. (2011)
